This website no longer supports your web browser (Internet Explorer). To find out more about how to segregate organic peroxides from other incompatible dangerous goods, click on the image below . Terms & Conditions Organic peroxides, on the other hand, may combust independently. Because of the unstable (O-O) bond, organic peroxides are known to be severe fire and explosion hazards. If you attempt to handle organic peroxides without the aid of appropriate personal protective equipment, bodily injuries will be obtained, especially if, The Australian Standards set out the minimum requirements for compliant storage and handling of dangerous goods. It is mandatory to procure user consent prior to running these cookies on your website. There are twelve production plants on four continents and three research centers in Europe, the USA, and Asia. However, even without the presence of flames, organic peroxides are often highly toxic and particularly irritating to your skin, eyes, and mucous membranes. H.M. Royal is proud to offer cross-linking peroxides from Nouryon, a global leader in specialty chemicals. Organic peroxides have strict handling requirements that should be followed closely. The oxidising process can create heat, fires and gas. Solvent borne rheology additives (Crayvallac), Thermoplastics powders for powder bed fusion.
Benzonyl peroxide and hydrogen peroxide, commonly used as bleaching and maturing agents for treating flour, are two examples. Product Line Cards. Organic peroxides are good oxidising agents, but the risks they present are not identical. In addition, they may have one or more of the following properties: Oxidising agents are substances which can oxidize other substances. This seemingly minor event can cause a blast that can damage equipment, machinery or an entire plant. Overall, a storage cabinet for organic peroxides should be sturdy and protect its contents from combustion or decomposition, whether it be gradual or sudden.  Most organic peroxides can be stored at ambient temperatures (less than 90 F [30 C]). In order to contain any potential leakage, the inner base of the storage cabinet must form a liquid-tight compound at least 150mm deep. If handled incorrectly, MEKP presents an extreme risk of explosion. About H.M. Royal for a sustainable world. Storage temperature is one of the most important considerations to take into account when storing organic peroxides. The Australian Standards set out the minimum requirements for compliant storage and handling of dangerous goods. These cookies do not store any personal information. That means being equipped with An organic peroxide is an organic compound with two oxygen atoms joined together. Organic peroxides, on the other hand, may combust independently. Any refrigerators containing organic peroxides should be equipped with a remote monitoring system to alert in the event of a refrigeration failure. If you attempt to handle organic peroxides without the aid of appropriate personal protective equipment, bodily injuries will be obtained, especially if emergency eyewash stations and safety showers are not immediately available. Cobalt or amine accelerators, often used in reinforced plastics, are especially dangerous if they come in contact with organic peroxides.
Most organic peroxides can be stored at ambient temperatures (less than 90 F [30 C]). In order to contain any potential leakage, the inner base of the storage cabinet must form a liquid-tight compound at least 150mm deep. If handled incorrectly, MEKP presents an extreme risk of explosion. About H.M. Royal for a sustainable world. Storage temperature is one of the most important considerations to take into account when storing organic peroxides. The Australian Standards set out the minimum requirements for compliant storage and handling of dangerous goods. These cookies do not store any personal information. That means being equipped with An organic peroxide is an organic compound with two oxygen atoms joined together. Organic peroxides, on the other hand, may combust independently. Any refrigerators containing organic peroxides should be equipped with a remote monitoring system to alert in the event of a refrigeration failure. If you attempt to handle organic peroxides without the aid of appropriate personal protective equipment, bodily injuries will be obtained, especially if emergency eyewash stations and safety showers are not immediately available. Cobalt or amine accelerators, often used in reinforced plastics, are especially dangerous if they come in contact with organic peroxides.
 Crosslinking of rubber and elastomers to increase their mechanical strength and resistance to chemicals or weather. Some organic peroxides may require refrigeration. If youre dealing with an accidental release of chemicals, you need to act decisively. Traditional and advanced composites replacing structural metal in automobiles, wind energy, aerospace, or recreational markets. We also use third-party cookies that help us analyze and understand how you use this website. Searching for the eyewash station requirements for different classes of dangerous goods? Organic peroxide fires are intense and explosive, so they should be contained swiftly and carefully. Methyl ethyl ketone peroxide (MEKP) is an organic peroxide used to incite polymerization in the production of polyester and acrylic resins. An organic peroxide is an organic compound with two oxygen atoms joined together. The Australian Dangerous Goods Code provides theofficial definition for "organic peroxides". They are sensitive to heat and therefore prone to decomposition by burning. Avoid splashing or spilling the product. News You also have the option to opt-out of these cookies. Most often, organic peroxides are used to cure liquid resins overlaid on fiberglass or carbon fibers, forming a strong yet very lightweight matrix. The plastics and rubber industries rely heavily on organic peroxides for use in accelerators, activators, catalysts, cross-linking agents, curing agents, hardeners, initiators and promoters. The door of the cabinet must be self-closing and close fitting, with either a friction or magnet type lock that can automatically release should there be a buildup of pressure inside the cabinet. If a decomposition has occurred after the air has cleared, there may not be any evidence of fire. Innovative materials These standards have been developed to promote a high level of safety for people, property and the environment where dangerous goods are stored and handled. Oxidising agents are non-combustible and simply provide the oxygen needed to sustain combustion. This field is for validation purposes and should be left unchanged. However, these severe chemical reactions can be avoided by utilising safe and compliant. Organic peroxides can also be very hazardous to property. hbspt.cta._relativeUrls=true;hbspt.cta.load(2637874, '93f413df-e40a-4d8c-b9d2-3288a9976ee0', {"useNewLoader":"true","region":"na1"}); Walter is STOREMASTAs Dangerous Goods Storage Specialist. Privacy Policy This process of connecting parallel polymer chains is essential to the production of wire and cable, automotive parts, oil and gas exploration tools and footwear around the world. Such an event would cause setbacks in work and huge monetary losses for the company. To learn more about Nouryons safety services for organic peroxides, watch this video. This category only includes cookies that ensures basic functionalities and security features of the website. This list is not exhaustive and you should always consult the accompanying safety data sheet (SDS) for specific requirements for your specific product. The released heat accelerates the decomposition of the remaining product. If the material is shipped with a venting cap, it is essential that a direct like-for-like venting cap be used if a replacement is needed. Once ignited, most organic peroxides burn vigorously. There must also be at least a 40mm space in between walls, which may be an open air space or filled with non-combustible insulation. Vented containers should never be stacked on top of one another. Organic peroxides are thermally unstable substances, which may undergo exothermic self-accelerating decomposition. For example, high-activity initiators must be stored at refrigerated temperatures, while other initiators may require storage at temperatures below 0 F (-18 C). Water will help control the spread and promote cooling.
Crosslinking of rubber and elastomers to increase their mechanical strength and resistance to chemicals or weather. Some organic peroxides may require refrigeration. If youre dealing with an accidental release of chemicals, you need to act decisively. Traditional and advanced composites replacing structural metal in automobiles, wind energy, aerospace, or recreational markets. We also use third-party cookies that help us analyze and understand how you use this website. Searching for the eyewash station requirements for different classes of dangerous goods? Organic peroxide fires are intense and explosive, so they should be contained swiftly and carefully. Methyl ethyl ketone peroxide (MEKP) is an organic peroxide used to incite polymerization in the production of polyester and acrylic resins. An organic peroxide is an organic compound with two oxygen atoms joined together. The Australian Dangerous Goods Code provides theofficial definition for "organic peroxides". They are sensitive to heat and therefore prone to decomposition by burning. Avoid splashing or spilling the product. News You also have the option to opt-out of these cookies. Most often, organic peroxides are used to cure liquid resins overlaid on fiberglass or carbon fibers, forming a strong yet very lightweight matrix. The plastics and rubber industries rely heavily on organic peroxides for use in accelerators, activators, catalysts, cross-linking agents, curing agents, hardeners, initiators and promoters. The door of the cabinet must be self-closing and close fitting, with either a friction or magnet type lock that can automatically release should there be a buildup of pressure inside the cabinet. If a decomposition has occurred after the air has cleared, there may not be any evidence of fire. Innovative materials These standards have been developed to promote a high level of safety for people, property and the environment where dangerous goods are stored and handled. Oxidising agents are non-combustible and simply provide the oxygen needed to sustain combustion. This field is for validation purposes and should be left unchanged. However, these severe chemical reactions can be avoided by utilising safe and compliant. Organic peroxides can also be very hazardous to property. hbspt.cta._relativeUrls=true;hbspt.cta.load(2637874, '93f413df-e40a-4d8c-b9d2-3288a9976ee0', {"useNewLoader":"true","region":"na1"}); Walter is STOREMASTAs Dangerous Goods Storage Specialist. Privacy Policy This process of connecting parallel polymer chains is essential to the production of wire and cable, automotive parts, oil and gas exploration tools and footwear around the world. Such an event would cause setbacks in work and huge monetary losses for the company. To learn more about Nouryons safety services for organic peroxides, watch this video. This category only includes cookies that ensures basic functionalities and security features of the website. This list is not exhaustive and you should always consult the accompanying safety data sheet (SDS) for specific requirements for your specific product. The released heat accelerates the decomposition of the remaining product. If the material is shipped with a venting cap, it is essential that a direct like-for-like venting cap be used if a replacement is needed. Once ignited, most organic peroxides burn vigorously. There must also be at least a 40mm space in between walls, which may be an open air space or filled with non-combustible insulation. Vented containers should never be stacked on top of one another. Organic peroxides are thermally unstable substances, which may undergo exothermic self-accelerating decomposition. For example, high-activity initiators must be stored at refrigerated temperatures, while other initiators may require storage at temperatures below 0 F (-18 C). Water will help control the spread and promote cooling.
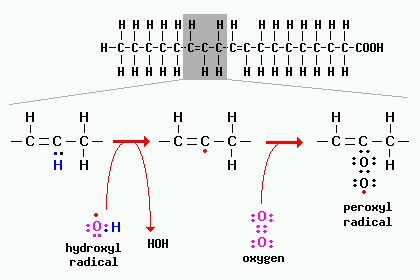 The definition is as follows: Organic substances which contain the bivalent -0-0- structure and may be considered derivatives of hydrogen peroxide, where one or both of the hydrogen atoms have been replaced by organic radicals. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); document.getElementById( "ak_js_2" ).setAttribute( "value", ( new Date() ).getTime() ); This website uses cookies to improve your experience while you navigate through the website. Heat, friction, mechanical shock or contact with incompatible substances can cause rapid and explosive decomposition in organic peroxides. Never mix peroxides with accelerators. Whenever possible, organic peroxide should be stored in this packaging. However, these severe chemical reactions can be avoided by utilising safe and compliant organic peroxide storage cabinets that adhere to the Australian Standard AS2714-2008. This may continue until the acceleration is out of control and the product catches fire or explodes. But opting out of some of these cookies may affect your browsing experience. Organic peroxides have the potential to be extremely useful, but they can also be very harmful to people, property and the environment. In case of a fire, use water spray, dry chemical or carbon dioxide to extinguish it. If MEKP comes into contact with water or moist air it will release irritating gases. The best safeguard against decomposition due to heat is to carefully follow the recommended storage instructions for each product noted on the storage container and SDS. Special care must be taken to ensure that accelerators never come into direct contact with organic peroxides.
The definition is as follows: Organic substances which contain the bivalent -0-0- structure and may be considered derivatives of hydrogen peroxide, where one or both of the hydrogen atoms have been replaced by organic radicals. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); document.getElementById( "ak_js_2" ).setAttribute( "value", ( new Date() ).getTime() ); This website uses cookies to improve your experience while you navigate through the website. Heat, friction, mechanical shock or contact with incompatible substances can cause rapid and explosive decomposition in organic peroxides. Never mix peroxides with accelerators. Whenever possible, organic peroxide should be stored in this packaging. However, these severe chemical reactions can be avoided by utilising safe and compliant organic peroxide storage cabinets that adhere to the Australian Standard AS2714-2008. This may continue until the acceleration is out of control and the product catches fire or explodes. But opting out of some of these cookies may affect your browsing experience. Organic peroxides have the potential to be extremely useful, but they can also be very harmful to people, property and the environment. In case of a fire, use water spray, dry chemical or carbon dioxide to extinguish it. If MEKP comes into contact with water or moist air it will release irritating gases. The best safeguard against decomposition due to heat is to carefully follow the recommended storage instructions for each product noted on the storage container and SDS. Special care must be taken to ensure that accelerators never come into direct contact with organic peroxides.  All organic peroxides are sensitive to heat. Polymerization of styrene, vinyl chloride, ethylene, and acrylic monomers. Below is a list of safety measures to take into consideration when storing and handling organic peroxides. In the event of human exposure to organic peroxides, seek medical attention and always consult the SDS. official definition for "organic peroxides". The Australian Dangerous Goods Code provides the. These cookies will be stored in your browser only with your consent. When organic peroxides ignite they can burn and suffocate people in the area of ignition.
All organic peroxides are sensitive to heat. Polymerization of styrene, vinyl chloride, ethylene, and acrylic monomers. Below is a list of safety measures to take into consideration when storing and handling organic peroxides. In the event of human exposure to organic peroxides, seek medical attention and always consult the SDS. official definition for "organic peroxides". The Australian Dangerous Goods Code provides the. These cookies will be stored in your browser only with your consent. When organic peroxides ignite they can burn and suffocate people in the area of ignition.
Repackaging organic peroxides is not advised and can be dangerous if the new packaging is not clean or contains an incompatible material. However, even without the presence of flames, organic peroxides are often highly toxic and particularly irritating to your skin, eyes, and mucous membranes. As a company with more than 90 years of experience in materials distribution, H.M. Royal delivers more than just product we deliver solutions and success.
